About Us
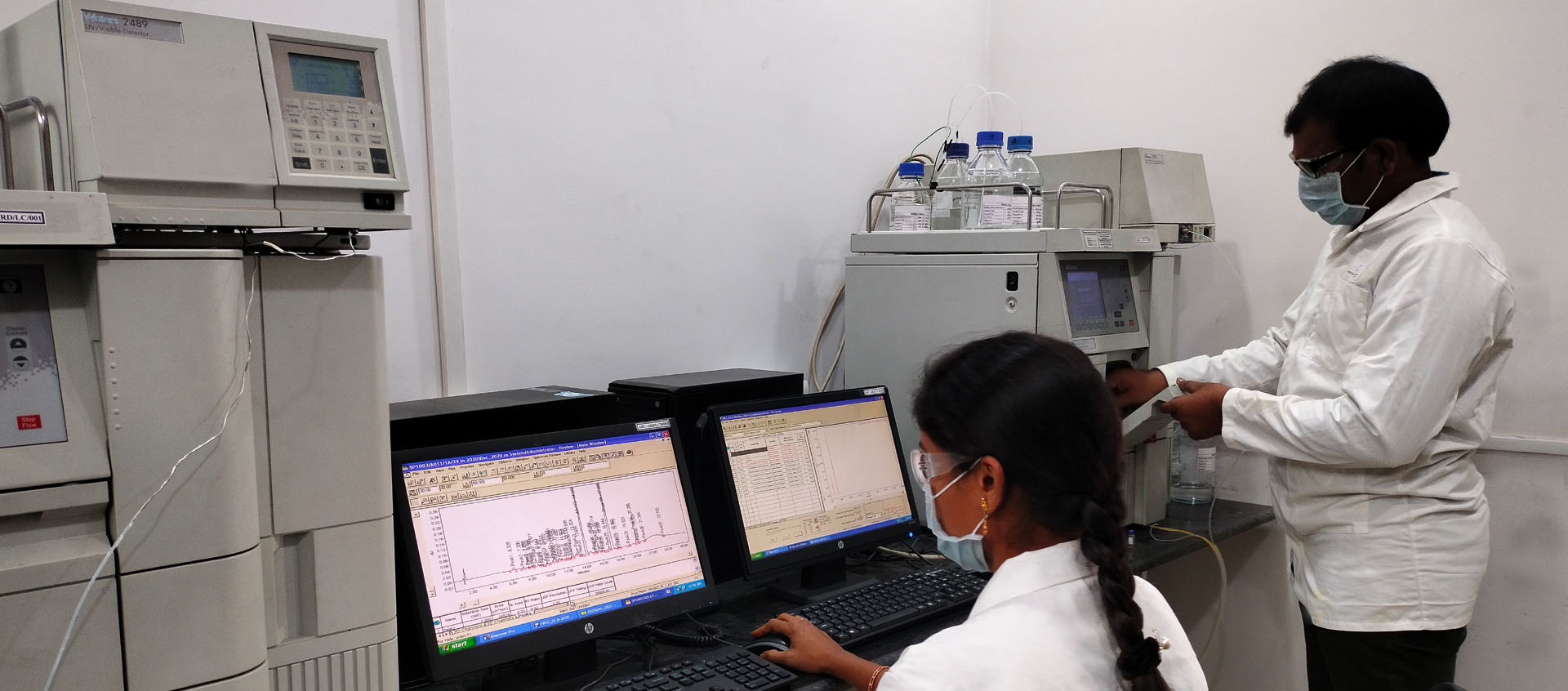
Enduring value for human life, Arouse Pharma Private Limited is a start-up pharmaceutical company based out of Hyderabad and promoted by highly talented pool of scientists. Innovative Research and Development is the backbone of Arouse Pharma Private Limited with customers at the core and employees at the front is our way of doing.
Expertize in
Developing and optimizing cost-effective synthetic processes for APIs and intermediates both for use in commercial and clinical studies.